Important terms are of course defined in ISO 9000 – the definition of the term ‘quality’, which most of you have probably come across before, serves as an example here:
‘3.6.2 Quality: the degree to which a set of inherent characteristics (3.10.1) of an object (3.6.1) fulfils requirements (3.6.4).’
In order to understand what is meant by ‘object’ here, a further explanation follows directly:
‘3.6.1 Object: entity, object – something perceptible or imaginable | EXAMPLE product (3.7.6), service (3.7.7), process (3.4.1), person, organization (3.2.1), system (3.5.1), resource.’
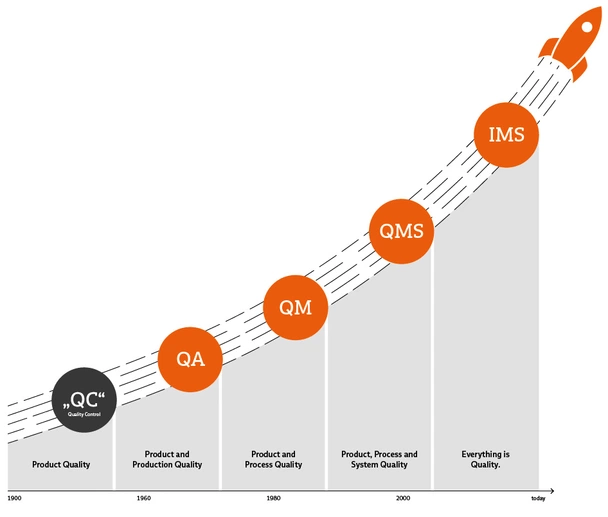
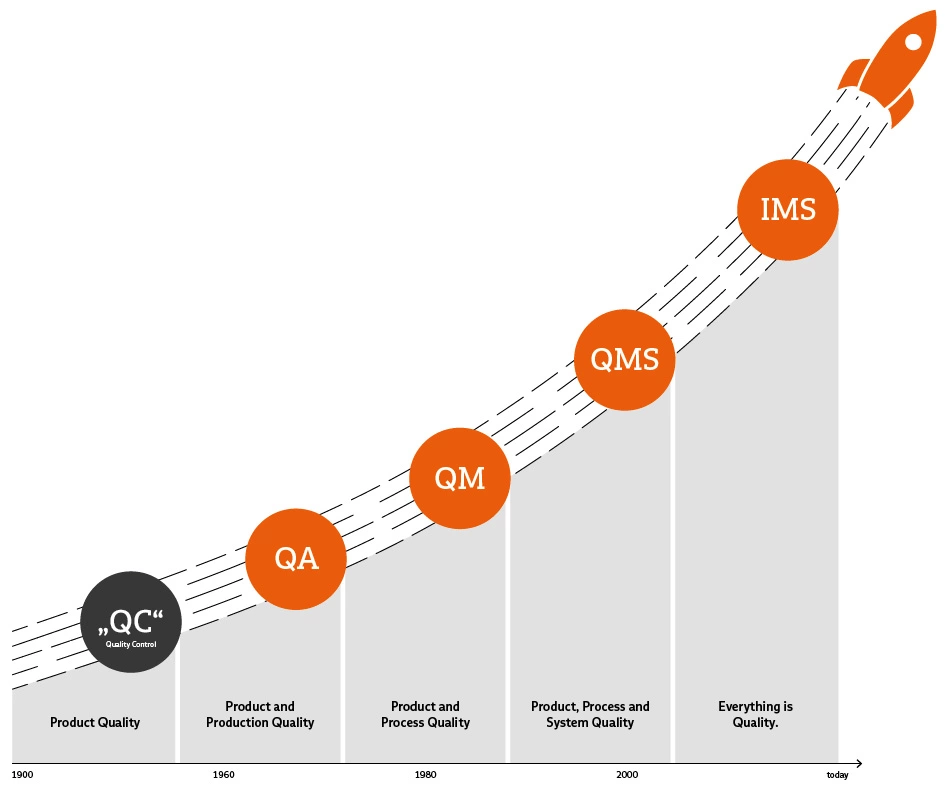
You can understand this if you take a very close look at the topic of quality. However, these definitions are certainly not accessible and easy to understand for all employees in the company. For this reason, we would like to try to categorize the terms as clearly as possible here – from QA and QM to QMS and IMS.







Comments
No comments